

At Daundi Biological Pvt Ltd, our purpose goes beyond just manufacturing medicines—we aim to make a meaningful difference in healthcare outcomes across India and beyond.

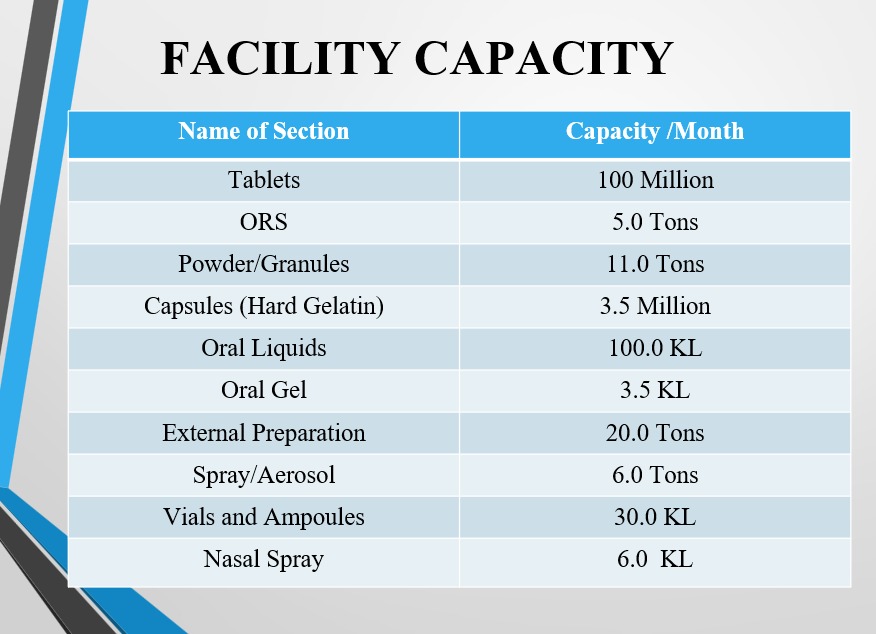
The manufacturing block delivers a broad range of formulations including tablets, syrups, creams, and injectables—all under controlled environments with stringent SOPs and quality protocols.
What sets Daundi apart is our integration of advanced infrastructure with continuous research-driven innovation. Our two-block facility is a unique combination of world-class manufacturing and a purpose-built R&D hub.
DBPL is fortunate to have a highly skilled chemist, along with specialists in quality control and assurance, as well as production design. Their deep understanding of both Indian and internationally acclaimed codes and pharmaceutical practices significantly enhances our ability to deliver top-notch products. Their commitment to excellence and adherence to stringent quality standards truly sets our operations apart.